Although hydrogen is the element found in the greatest quantities in the universe, it doesn't exist in a purely natural state on earth as fossil fuels do. It is attractive as a fuel because, by a process called electrolysis, it can theoretically be derived from water, the air, and many other substances. Of course, until ways can be found to fill a vehicle's tank with water and have it produce its own hydrogen, there's still the problem of the fossil-fuel energy necessary to produce hydrogen in large quantities and deliver it to a vehicle as a liquid or gas. And because hydrogen is highly flammable and very cold in its liquid form (minus 453°F!), there are safety concerns about shipping it, pumping it into a vehicle's fuel tank, and storing it there under extreme pressure.
How Fuel Cells Work
A fuel cell creates electrical current from hydrogen and oxygen and may well be the best use of hydrogen as an alternative to fossil-fueled vehicles. When used in a hydrogen-powered EV called a fuel cell vehicle (FCV), virtually the only tailpipe emissions that hydrogen produces are water molecules. Although there are many types of fuel cells, the ones using a polymer exchange membrane are the most popular for vehicles because they produce more power and operate at lower temperatures, which enables them to warm up faster than other, hotter, types of fuel cells. The resulting current is fed directly to the vehicle's electric motor.
As you can see in Figure 10-6, a fuel cell has a positive anode and a negative cathode. Hydrogen gas enters the cell and is sent to the anode, while oxygen in the form of air flows to the cathode. A thin plate of platinum particles serves as a catalyst to split each atom of hydrogen into positive and negative ions. The polymer electrolyte membrane lets the positive ions go through it to the anode. The negative ions can't get through and have to take another route to the cathode — on the way they create electrical current to drive the electric motor of the vehicle (represented as a light bulb in Figure 10-6).
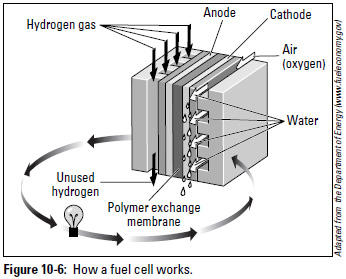
When the positive and negative hydrogen ions are reunited at the cathode, they combine with the oxygen from the air into H2O and leave the cell as water vapor. On vehicles, hundreds of individual fuel cells are linked together into fuel cell stacks to increase the electrical output. Whether or not they become widely available depends on whether cheaper and more efficient alternative vehicles eliminate the need for them.
From Auto Repair for Dummies, copyright © 2009 by Wiley Publishing, Inc., Indianapolis, Indiana. Used by arrangement with John Wiley & Sons, Inc.










